Integrating chemistry, molecular biology and experimental oncology
TARGETING “UNDRUGGABLE” CANCERS
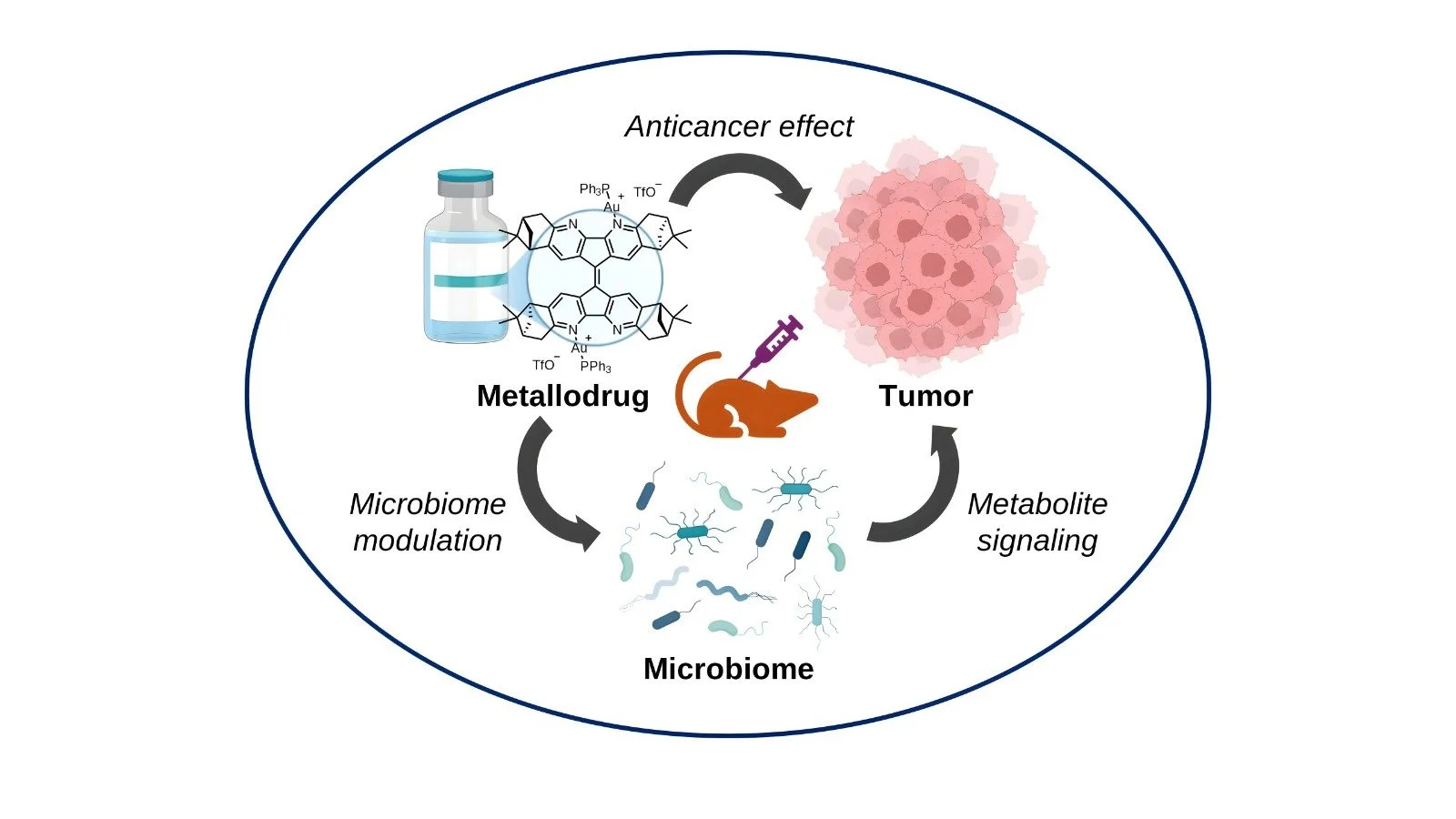
1) Targeting the Microbiome for Cancer Therapy
Our lab investigates how the microbiome influences cancer progression and therapy, with the long-term goal of developing microbiome-targeted treatments that are precise and clinically translatable. Although the microbiome’s role in cancer has only recently emerged, we view it as a rich, tunable axis for intervention — one that can complement and improve existing therapeutic strategies despite the well-recognized challenges of moving from in vitro observations to patient care.
We focus on controlled manipulation of microbial communities as a therapeutic strategy: identifying microbe-drug and microbe-host interactions, screening microbiome-active compounds, and using rational design approaches to create interventions that shift microbial composition or metabolism in ways that enhance anticancer efficacy. Our recent work demonstrates that, even given the microbiome’s multifactorial complexity, targeted compositional changes can be a viable route for drug discovery and development.
Ultimately the lab aims to translate these discoveries into tailored microbiome-based therapies that increase treatment accuracy and effectiveness for individual patients, by combining mechanistic microbiology, preclinical modeling, and design-driven screening pipelines.
Babak M.V et al. Proc. Natl. Acad. Sci., 122, 44, e2417269122, 2025
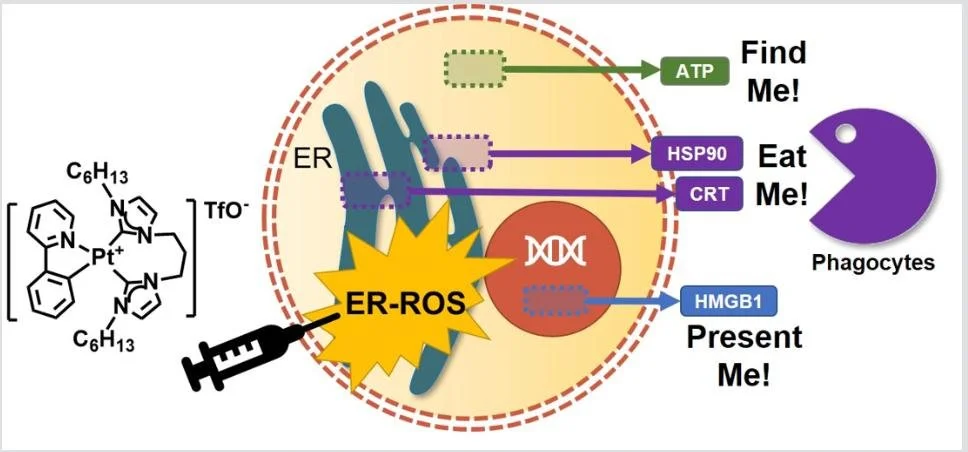
2) Activation of the Immune System
Toxic chemotherapy inflicts further distress in patients already suffering from the disease, forcing some of them to opt out of their treatment plans. Prevention of adverse events or more effective management of adverse and collateral effects arising from anticancer drugs will continue to be an area of urgent need. While chemotherapy aims to eradicate cancer cells, immunotherapy activates the immune system of the patient to ensure clearance of cancer cells and establishment of long-term immunological memory. We aim to develop experimental drugs that will either educate immune cells to recognize and eradicate cancer invaders or activate “immunological visibility” of cancer cells by forced release of “eat me” and “find me” signals.
Tham M.J.R., Babak M.V, Ang W.H. Angewandte Chemie Int. Ed., 59(43), 19070-19078, 2020
3) AI-assisted Metallodrug Discovery
Our lab develops a compact, AI-driven discovery platform for metal-based anticancer agents across diverse transition metals. We confront coordination-specific machine-learning challenges-variable oxidation states, coordination geometries, and metal-ligand interactions-through rigorous data curation, metal-aware featurization (e.g., formal oxidation state, coordination number, donor-atom types and denticity), and tailored ML architectures for supervised prediction and generative design.
Model outputs both prioritize candidates and propose de novo scaffolds that enter an iterative experimental loop of synthesis, in vitro and in vivo evaluation, and mechanistic profiling. Our early proof-of-concept studies show strong concordance between predicted and observed activity, supporting a scalable pipeline to accelerate lead identification and address cancer resistance.
Babak M.V. et al. ChemRxiv, 10.26434/chemrxiv-2025-pp32k.